Respiratory Care Devices Market is expected to generate a revenue of USD 40.31 Billion by 2031, Globally, at 5.89% CAGR: Verified Market Research®
The Respiratory Care Devices Market presents robust growth opportunities driven by rising chronic respiratory conditions, technological innovations, and aging populations demanding homecare solutions. However, high equipment costs, reimbursement challenges, and safety concerns in non-clinical settings pose significant barriers. North America's dominance—fueled by strong infrastructure and supportive policies—makes it a prime entry point. For new entrants and existing players, success hinges on localized strategies: offering cost-effective, user-friendly devices in emerging markets, leveraging telehealth integration, and aligning product portfolios with regional reimbursement frameworks to maximize adoption and long-term market penetration.
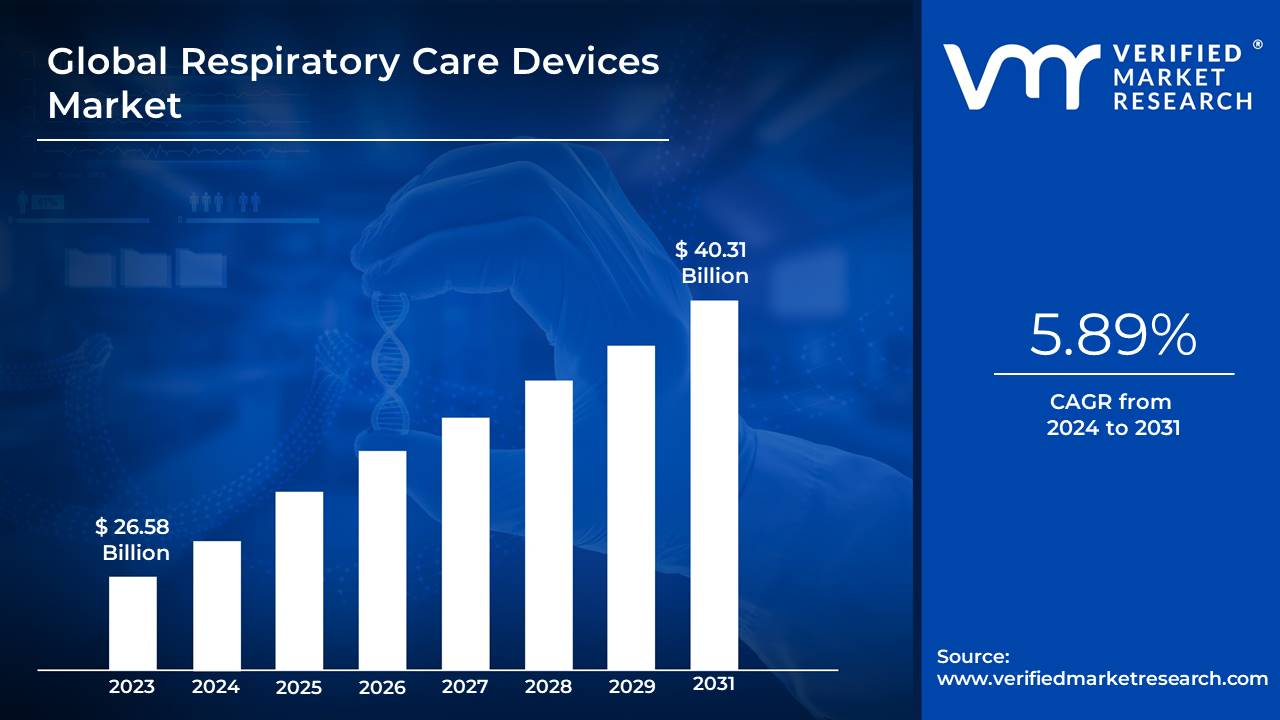
Lewes, Delaware, July 21, 2025 (GLOBE NEWSWIRE) -- The Global Respiratory Care Devices Market Size is projected to grow at a CAGR of 5.89% from 2024 to 2031, according to a new report published by Verified Market Research®. The report reveals that the market was valued at USD 26.58 Billion in 2023 and is expected to reach USD 40.31 Billion by the end of the forecast period.
The global Respiratory Care Devices Market is witnessing notable expansion due to technological integration in respiratory support systems and increasing hospital admissions for pulmonary diseases. Rising awareness about homecare respiratory therapy is further fueling demand across developed and emerging economies.
Key Highlights of the Report:
- Market Size & Forecast: In-depth analysis of current value and future projections
- Segment Analysis: Breaks down the market by product, and End User for focused strategy development.
- Regional Insights: Comprehensive coverage of North America, Europe, Asia-Pacific, and more
- Competitive Landscape: Profiles key players, their strategic initiatives, and innovation-driven growth approaches.
- Growth Drivers & Challenges: Analyzes the forces accelerating growth and the restraints hindering large-scale adoption.
- Challenges and Risk Assessment: Evaluates ethical debates, off-target effects, and regulatory complexities.
Why This Report Matters:
This report equips stakeholders with critical market intelligence to capitalize on high-growth areas, anticipate regulatory shifts, and identify product development opportunities in the respiratory care space. Backed by verified data and expert analysis, it enables strategic decision-making for manufacturers, investors, and healthcare providers.
Who You Should Read This Report:
- Medical device manufacturers & suppliers
- Hospital procurement heads & clinicians
- B2B investors & venture capitalists
- Healthcare technology innovators
- Regulatory agencies & policy analysts
- Strategic consultants & business analysts
 For more information or to purchase the report, please contact us at: https://www.verifiedmarketresearch.com/download-sample/?rid=23929
For more information or to purchase the report, please contact us at: https://www.verifiedmarketresearch.com/download-sample/?rid=23929
Browse in-depth TOC on “Global Respiratory Care Devices Market Size”
202 - Pages
126 – Tables
37 – Figures
Report Scope
| REPORT ATTRIBUTES | DETAILS |
| Study Period | 2020-2031 |
| Base Year | 2023 |
| FORECAST PERIOD | 2024-2031 |
| Historical Period | 2020-2022 |
| KEY COMPANIES PROFILED | Philips Healthcare, Medtronic plc, ResMed, Inc., Fisher & Paykel Healthcare Corporation Limited, GE Healthcare, Drägerwerk AG & Co. KGaA |
| UNIT | Value (USD Billion) |
| SEGMENTS COVERED |
|
| CUSTOMIZATION SCOPE | Free report customization (equivalent up to 4 analyst’s working days) with purchase. Addition or alteration to country, regional & segment scope |
Global Respiratory Care Devices Market Overview
Market Driver
1. Rising Global Prevalence of Chronic Respiratory Diseases: The increasing incidence and burden of chronic respiratory diseases (CRDs) such as Chronic Obstructive Pulmonary Disease (COPD), asthma, sleep apnea, and cystic fibrosis are pivotal drivers of demand for respiratory care devices. The World Health Organization (WHO) estimates that COPD alone is responsible for over 3 million deaths annually, making it the third leading cause of death globally. Asthma affects over 262 million people worldwide. With lifestyle factors such as smoking, occupational exposure to harmful dust and chemicals, and growing air pollution levels, especially in urban areas, the patient base for respiratory support continues to expand. As a result, there is a consistent demand for oxygen therapy devices, nebulizers, and ventilators to manage these chronic conditions both in clinical and homecare settings. This upward trend in respiratory diseases creates long-term demand for preventive, therapeutic, and monitoring solutions.
2. Technological Advancements in Respiratory Devices: Rapid innovation in respiratory technology is reshaping the landscape of patient care. Modern respiratory care devices are increasingly becoming compact, portable, and intelligent. Integration of advanced technologies such as artificial intelligence (AI), machine learning (ML), IoT, and cloud-based data management has enhanced device functionality, accuracy, and usability. For instance, smart inhalers now offer real-time dosage monitoring and patient adherence tracking, which helps healthcare providers personalize treatment plans. AI-powered ventilators automatically adjust oxygen levels and air pressure based on patient breathing patterns, improving outcomes and reducing dependency on clinical staff. Moreover, portable oxygen concentrators and battery-operated CPAP machines have become vital tools in enhancing mobility for patients who need respiratory support outside clinical environments. These innovations not only improve patient outcomes and quality of life but also encourage the adoption of devices across broader market segments.
3. Surge in Geriatric Population and Shift Toward Homecare Settings: The global demographic shift toward an aging population is significantly contributing to the demand for respiratory care solutions. Elderly individuals are more susceptible to respiratory conditions due to declining lung function and a higher prevalence of comorbidities such as cardiovascular diseases, diabetes, and neurological disorders. According to the United Nations, by 2050, 1 in 6 people globally will be over age 65, up from 1 in 11 in 2019. This demographic trend is driving the need for long-term respiratory support systems. Additionally, the rising preference for home-based care—driven by factors such as lower hospitalization costs, enhanced patient comfort, and post-COVID-19 safety concerns—is leading to increased adoption of compact, easy-to-use homecare devices like CPAP, BiPAP machines, and portable nebulizers. Medical device companies are now focused on developing patient-friendly, plug-and-play solutions to cater to the growing homecare market segment.
 To Purchase a Comprehensive Report Analysis: https://www.verifiedmarketresearch.com/select-licence/?rid=23929
To Purchase a Comprehensive Report Analysis: https://www.verifiedmarketresearch.com/select-licence/?rid=23929
Market Restraint
1. High Cost of Advanced Respiratory Equipment: The adoption of advanced respiratory care devices is often hindered by high capital costs, especially in resource-limited settings. High-end ventilators, portable oxygen concentrators, and digital CPAP machines entail significant upfront investment and often require specialized training for effective operation. Furthermore, associated costs such as consumables (filters, tubing, masks), device calibration, maintenance, and software upgrades add to the total cost of ownership. These financial challenges become particularly critical in low-income countries, smaller clinics, and rural healthcare centers that operate under tight budget constraints. Even in developed markets, the affordability of such devices is often a concern for uninsured or underinsured patients. The economic burden may disincentivize both healthcare providers and end-users from fully adopting technologically advanced solutions, thereby slowing market penetration and expansion.
2. Limited Reimbursement Coverage in Developing Markets: Another significant barrier to growth is the lack of standardized and comprehensive reimbursement policies for respiratory care devices in many parts of Asia, Africa, and Latin America. In several developing countries, national health insurance schemes do not adequately cover outpatient services or homecare respiratory equipment. Even in regions with insurance coverage, bureaucratic delays, inconsistent policies, and limited provider networks restrict patient access to critical respiratory support. As a result, many patients are forced to pay out-of-pocket, which can lead to treatment discontinuation or avoidance altogether. This issue not only affects patient health outcomes but also undermines the business case for medical device manufacturers to expand into these underserved markets. Addressing these policy gaps is critical for ensuring equitable access and for driving long-term commercial growth in emerging economies.
3. Safety Concerns and Improper Usage in Homecare Settings: While the homecare segment represents a lucrative opportunity for respiratory care device manufacturers, it also introduces risks that can negatively impact market growth. Without proper training or oversight, patients and caregivers may misuse devices, leading to incorrect dosages, poor maintenance, and delayed response to critical health changes. For example, improper use of CPAP machines can lead to nasal congestion, mask leaks, or discomfort, discouraging continued use. Similarly, unsanitary nebulizers or concentrators can become breeding grounds for infections if not cleaned correctly. These concerns not only pose threats to patient safety but can also lead to legal and regulatory scrutiny. Moreover, device failure due to power outages or lack of technical support in remote areas can compromise therapy outcomes. As a result, apprehension regarding the reliability and usability of homecare devices remains a considerable restraint, particularly in regions lacking adequate health literacy and infrastructure.
Geographical Dominance: North America holds the dominant share in the Respiratory Care Devices Market, driven by high prevalence of respiratory disorders, robust healthcare infrastructure, and early adoption of advanced technologies. The presence of leading market players and favorable reimbursement policies further accelerate market penetration. Moreover, rising geriatric population and increasing awareness regarding home-based respiratory therapy contribute significantly to sustained regional demand, positioning North America as a critical revenue-generating hub for global stakeholders.
Key Players
The “Global Respiratory Care Devices Market” study report will provide a valuable insight with an emphasis on the global market. The major players in the market are Philips Healthcare, Medtronic plc, ResMed, Inc., Fisher & Paykel Healthcare Corporation Limited, GE Healthcare, Drägerwerk AG & Co. KGaA.
Respiratory Care Devices Market Segment Analysis
Based on the research, Verified Market Research has segmented the global market into Product, End-User, and Geography.
-
Respiratory Care Devices Market, by Product
- Therapeutic Devices
- Monitoring Devices
- Consumables
- Accessories
-
Respiratory Care Devices Market, by End-User
- Hospitals
- Homecare Setting
-
Respiratory Care Devices Market, by Geography
-
North America
- U.S
- Canada
- Mexico
-
Europe
- Germany
- France
- U.K
- Rest of Europe
-
Asia Pacific
- China
- Japan
- India
- Rest of Asia Pacific
-
ROW
- Middle East & Africa
- Latin America
-
North America
Browse Related Reports:
Global Respiratory Gas Blender Market Size By Type (Manual, Electronic), By Application (Hospitals, Ambulatory Surgical Centers), By Geography, And Forecast
Global BiPAP Machines Market Size By Type (BiPAP And BiPAP ST), By Application (NICU/PICU, Sleep Apnea And Acute Respiratory Failure), By End-User (Ambulatory Surgical Centers, Hospitals, and Home Care), By Geography, And Forecast
Global Catheter Stabilization Devices Market Size By Application (Cardiovascular Procedures, Respiratory Procedures, General Surgery), By Product Type (Chest Drainage Tube Securement Devices, Peripheral Securement Devices, Arterial Securement Devices), By End User (Hospitals, Home Care Settings, Emergency Clinics), By Geography, And Forecast
Global Neonatal Intensive Care Respiratory Devices Market Size By Device Type (Nebulizers, Inhalers, Ventilators), By End-User (NICU Hospitals, Specialty Clinics, Nursing Homes), By Geography, And Forecast
Top 7 Interventional Cardiology Device Companies pioneering innovations in heart health
Visualize Respiratory Care Devices Market using Verified Market Intelligence -:
Verified Market Intelligence is our BI Enabled Platform for narrative storytelling in this market. VMI offers in-depth forecasted trends and accurate Insights on over 20,000+ emerging & niche markets, helping you make critical revenue-impacting decisions for a brilliant future.
VMI provides a holistic overview and global competitive landscape with respect to Region, Country, Segment, and Key players of your market. Present your Market Report & findings with an inbuilt presentation feature saving over 70% of your time and resources for Investor, Sales & Marketing, R&D, and Product Development pitches. VMI enables data delivery In Excel and Interactive PDF formats with over 15+ Key Market Indicators for your market.
About Us
Verified Market Research® stands at the forefront as a global leader in Research and Consulting, offering unparalleled analytical research solutions that empower organizations with the insights needed for critical business decisions. Celebrating 10+ years of service, VMR has been instrumental in providing founders and companies with precise, up-to-date research data.
With a team of 500+ Analysts and subject matter experts, VMR leverages internationally recognized research methodologies for data collection and analyses, covering over 15,000 high impact and niche markets. This robust team ensures data integrity and offers insights that are both informative and actionable, tailored to the strategic needs of businesses across various industries.
VMR's domain expertise is recognized across 14 key industries, including Semiconductor & Electronics, Healthcare & Pharmaceuticals, Energy, Technology, Automobiles, Defense, Mining, Manufacturing, Retail, and Agriculture & Food. In-depth market analysis cover over 52 countries, with advanced data collection methods and sophisticated research techniques being utilized. This approach allows for actionable insights to be furnished by seasoned analysts, equipping clients with the essential knowledge necessary for critical revenue decisions across these varied and vital industries.
Verified Market Research® is also a member of ESOMAR, an organization renowned for setting the benchmark in ethical and professional standards in market research. This affiliation highlights VMR's dedication to conducting research with integrity and reliability, ensuring that the insights offered are not only valuable but also ethically sourced and respected worldwide.
Follow Us On: LinkedIn | Twitter | Threads | Instagram | Facebook

Mr. Edwyne Fernandes Verified Market Research® US: +1 (650)-781-4080 US Toll Free: +1 (800)-782-1768 Email: sales@verifiedmarketresearch.com Web: https://www.verifiedmarketresearch.com/ SOURCE – Verified Market Research®
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
